Activity 4 — Applying Coulomb’s Law
Consider two identical conducting spheres, one carrying charge +q and the other carrying charge +3q, that are initially held a distance d apart. The spheres are allowed to touch briefly and then returned to separation distance d. Is the magnitude of the force they exert on each other after touching greater than, less than, or the same as the magnitude of the force they exerted on each other before touching?
You might recall from PHYS 1111 Newton’s law of gravitation:
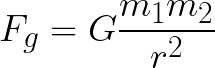
which looks a lot like Coulomb’s law, except that the interaction is between objects with mass rather than electric charge. If you consider the hydrogen atom, it is made of a proton (the nucleus) and an electron, that are bound together with the electric force. But the proton and electron both have mass, too. So there should be a gravitational attraction between them. I want you to compare these two forces: electrical and gravitational. You will need to look up some data, like the masses of the particles and the constants. Which force is bigger and by what factor?
Compare the magnitudes of the gravitational and electric forces exerted by the nucleus of a hydrogen atom on an electron when the two particles are 0.50 x 10-10 m apart.