Nuclear Reactions

13.1 Nuclear Reactions
Learning Objectives
- Describe and compare three types of nuclear radiation
- Use nuclear symbols to describe changes that occur during nuclear reactions
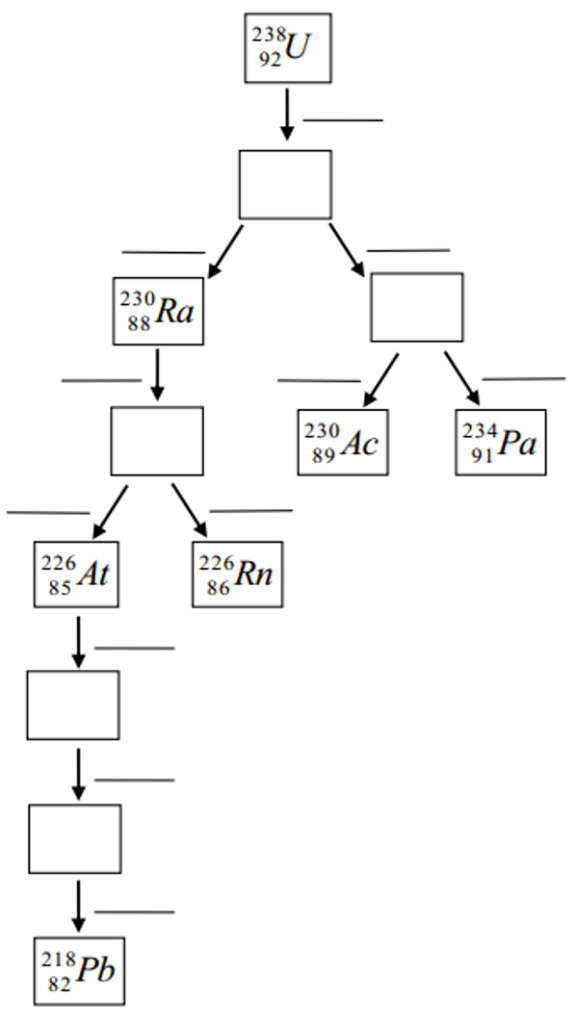
- Describe processes involved in the decay series of heavy elements
Alpha Decay
Beta Decay
Gamma Decay

Practice!
| Practice 13.1.1 |
|---|
| Which kinds of unstable nuclei typically decay by emitting an alpha particle? |
| A. those with too many neutrons |
| B. those with too many protons |
| C. those with too many neutrons and too many protons |
| D. Misleading question—the numbers of neutrons and protons in a nucleus are unrelated to whether or not it emits an alpha particle. |
| E. Misleading question—a nucleus cannot decay by emitting an alpha particle spontaneously. Alpha particles are released only in collisions between nuclei. |
| Practice 13.1.2 |
|---|
| Which kinds of unstable nuclei typically decay by emitting an electron? |
| A. those with too many neutrons |
| B. those with too many protons |
| C. those with too many neutrons and too many protons |
| D. Misleading question—the numbers of neutrons and protons in a nucleus are unrelated to whether or not it emits an electron. |
| E. Misleading question—a nucleus cannot decay by emitting an electron spontaneously. Electrons are released only in collisions between nuclei. |
| Practice 13.1.3 |
|---|
| Which kinds of unstable nuclei typically decay by emitting a gamma-ray photon? |
| A. those with too many neutrons |
| B. those with too many protons |
| C. those with too many neutrons and too many protons |
| D. Misleading question—the numbers of neutrons and protons in a nucleus are unrelated to whether or not it emits gamma rays. |
| E. Misleading question—a nucleus cannot decay by emitting gamma ray spontaneously. Gamma rays are released only in collisions between nuclei. |
| Practice 13.1.4 |
|---|
| Radon-222 is a gas produced by alpha decay. Its parent nuclide is |
| A. lead-220 |
| B. polonium-218 |
| C. radium-226 |
| D. thorium-224 |
| Practice 13.1.5 |
|---|
| What value of Z (atomic number) and A (mass number) result in the following β-decay? |
| A. Z = 5; A = 14 |
| B. Z = 4; A = 10 |
| C. Z = 6; A = 14 |
| D. Z = 7; A = 14 |
| E. Z = 7; A = 13 |
| Practice 13.1.6 |
|---|
| Which of the following is the correct daughter nucleus associated with the alpha decay of 157Hf? |
| A. 153Hf |
| B. 153Yb |
| C. 157Yb |
| Practice 13.1.7 |
|---|
| Which of the following is the correct daughter nucleus associated with the beta decay of 184Hf? |
| A. 183Hf |
| B. 183Ta |
| C. 184Ta |

Discuss!
A radioactive sample undergoes three different types of radioactive decays and emits three different types of particles.

The particles are emitted into a region of space with a uniform magnetic field directed out of the page and follow the paths indicated. None of the particles bend either into or out of the screen. For each path, identify the radioactive decay (α, β+, β-, γ) or state that the type of decay cannot be determined based on the information provided. Ignore the neutrinos (and antineutrinos) emitted in beta decay.
Complete the following hypothetical radioactive chain with the correct decay process on each line and the correct nucleus in each box.
23Ne decays to 23Na by negative beta emission. What is the maximum kinetic energy of the emitted electrons?