Relativistic Energy
| Practice 2.3.1 |
|---|
| What is the rest energy of an electron? |
| A. 8.19 MeV |
| B. 511 MeV |
| C. 0.409 MeV |
| D. 0.511 MeV |
| Practice 2.3.2 |
|---|
| If the rest of energy of one electron is used to accelerate a second electron, how fast will the second electron be moving? |
| A. 0.987c |
| B. 0.215c |
| C. 0.866c |
| D. 0.433c |
As a follow-up to practice problem 2.3.2, how much energy would be required to reach 0.99c?
| Practice 2.3.3 |
|---|
| If the total energy of a proton is three times its rest energy, what is the speed of the proton? |
| A. 0.94c |
| B. 0.46c |
| C. 0.75c |
| D. 0.99c |
| Practice 2.3.4 |
|---|
| If the total energy of a proton is three times its rest energy, what is the kinetic energy of the proton? |
| A. 664 MeV |
| B. 415 MeV |
| C. 1876 MeV |
| D. 963 MeV |
| Practice 2.3.5 |
|---|
| If the total energy of a proton is three times its rest energy, what is the momentum of the proton? |
| A. 2814 MeV/c |
| B. 2653 MeV/c |
| C. 1625 MeV/c |
| D. 812 MeV/c |
| Practice 2.3.6 |
|---|
| The following three particles all have the same total energy E: (a) a photon (b) a proton (c) an electron Rank the magnitudes of the particles’ momentum from smallest to greatest. |
| A. (b) < (a) < (c) |
| B. (c) < (b) < (a) |
| C. (b) < (c) < (a) |
| D. (a) < (c) < (b) |
The file below contains a table of atomic masses for the elements and their most abundant isotopes.
| Practice 2.3.7 |
|---|
| How does the mass of a hydrogen atom compare to the mass of a proton plus the mass of an electron? |
| A. It is the same: mH = me + mp |
| B. It is less: mH < me + mp |
| C. It is greater: mH > me + mp |
| Practice 2.3.8 |
|---|
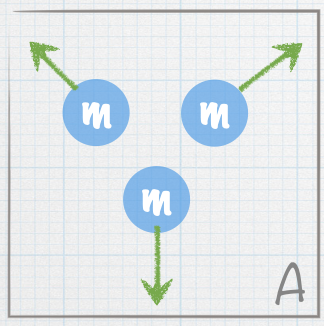
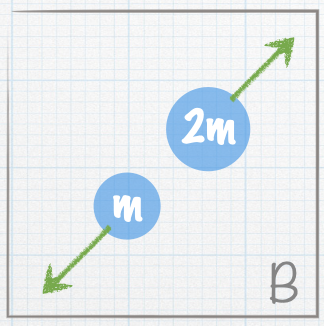
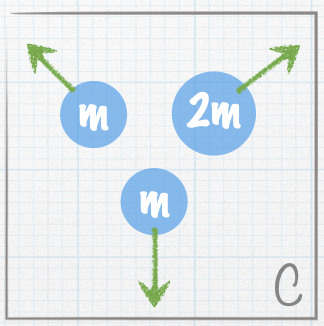
| A stationary nucleus of mass 3m decays into smaller nuclei. The masses of the smaller nuclei are given in the figures. The green arrows represent the velocities of the smaller nuclei. Which of the following represents a possible final state? |
 |
 |
 |
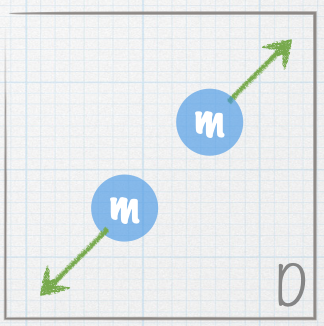 |
| E. Both A and B |
It is known that two oxygen atoms attract one another and can unite to form an O2 molecule, with the release of energy Eout ≈ 5 eV (in the form of light if the reaction takes place in isolation). By how much is the O2 molecule lighter than the two O atoms (in units of kg)?
Check your answer: ΔM ≈ 9 × 10-36 kg