Electron Spin

8.1 Electron Spin
Learning Objectives
- Express the state of an electron in a hydrogen atom in terms of five quantum numbers
- Use quantum numbers to calculate the magnitude and direction of the spin and magnetic moment of an electron
- Explain the fine and hyperfine structure of the hydrogen spectrum in terms of magnetic interactions inside the hydrogen atom

Practice!
| Practice 8.1.1 |
|---|
 Consider a magnet in an inhomogeneous field, as shown. Which way will the magnet feel a force? |
| A. up |
| B. down |
| C. left |
| D. right |
| E. no net force |
| Practice 8.1.2 |
|---|
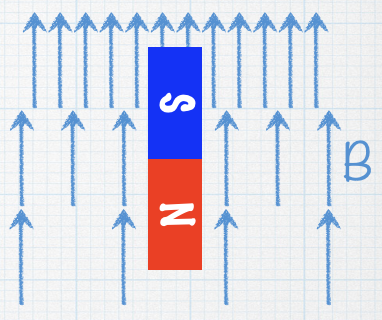 Consider a magnet in an inhomogeneous field, as shown. Which way will the magnet feel a force? |
| A. up |
| B. down |
| C. left |
| D. right |
| E. no net force |
| Practice 8.1.3 |
|---|
 Consider a magnet in an inhomogeneous field, as shown. Which way will the magnet feel a force? |
| A. up |
| B. down |
| C. left |
| D. right |
| E. no net force |

Discuss!
If you send a beam of bar magnets through this inhomogeneous magnetic field and detect them on the other side, what will the distribution look like?
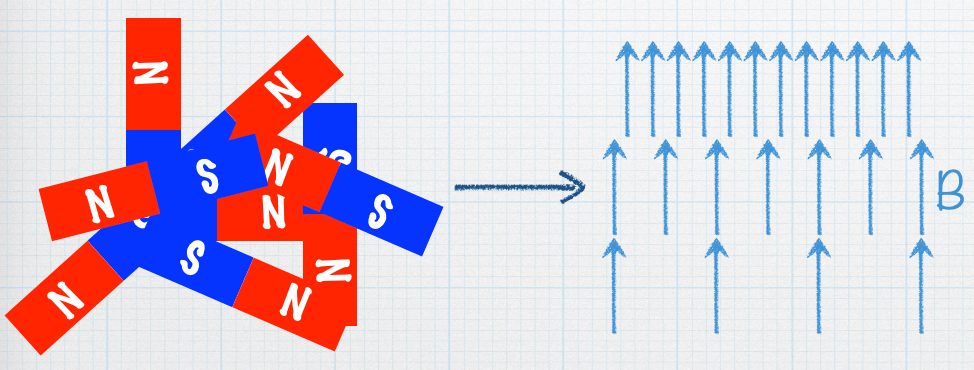
The Stern-Gerlach Experiment
Spin Angular Momentum

Practice!
| Practice 8.1.3 |
|---|
| In 1921, Stern and Gerlach performed an experiment that first demonstrated |
| A. orbital angular momentum quantization |
| B. energy quantization |
| C. space quantization |
| D. magnetic orbital quantization |
| E. that particles behave like waves |
| Practice 8.1.4 |
|---|
| The magnitude of the spin angular momentum for an electron is equal to |
A. 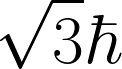 |
B.  |
| C. |
D. 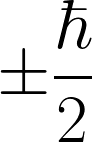 |
E. 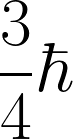 |