Lasers

9.2 Lasers
Learning Objectives
- Describe the physical processes necessary to produce laser light
- Explain the difference between coherent and incoherent light
- Describe the application of lasers to a CD and Blu-Ray player

Practice!
| Practice 9.2.1 |
|---|
Consider this glass tube full of atoms, like a discharge lamp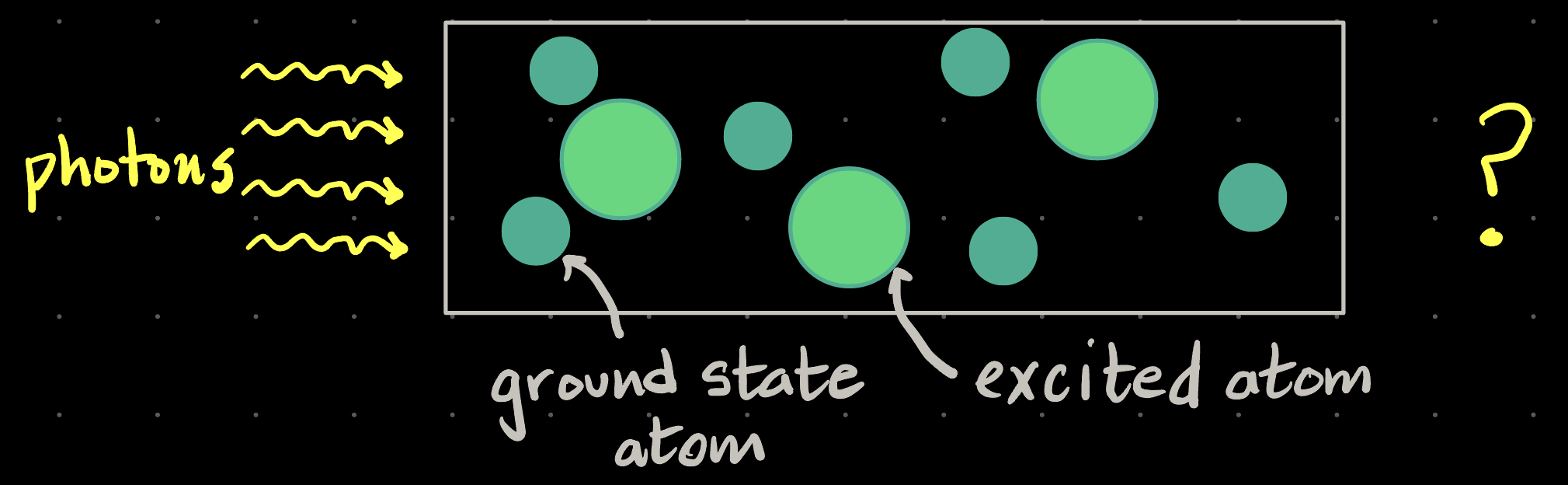 Compared to the number of photons entering the tube, we would expect that, on average: |
| A. more photons will come out the right hand end of tube |
| B. less will come out |
| C. same number as go in |
| D. none will come out. |

Practice!
| Practice 9.2.2 |
|---|
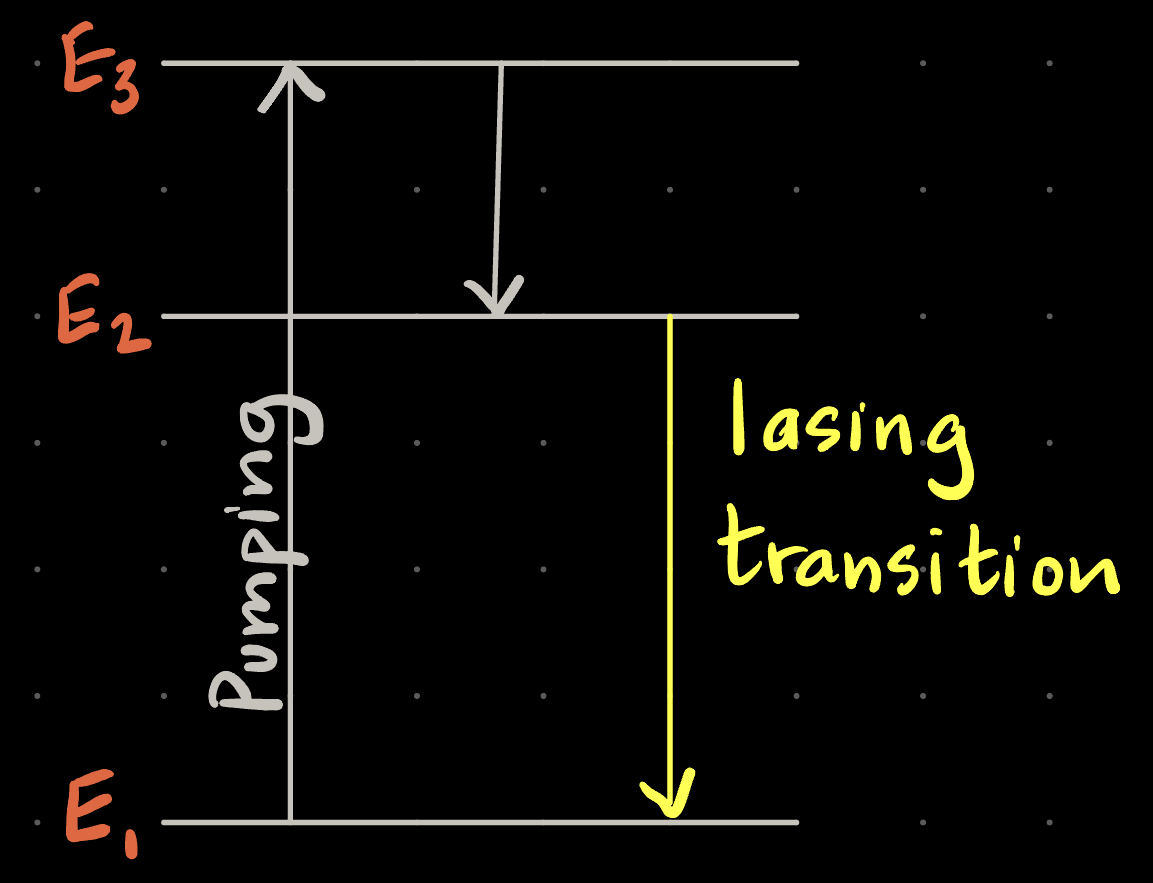 To create a population inversion between the ground state (E1) and E2, we would need: |
| A. The time spent in E3 (Δt3) before spontaneously transitioning to E2 to be long, and the time spent in E2 (Δt2) before transitioning to E1 to be short |
| B. Δt3 = Δt2 |
| C. Δt3 short, Δt2 long |
| D. Does not matter |

Discuss!
The figure below shows portions of the energy-level diagrams of the helium and neon atoms in a HeNe laser. An electrical discharge excites the He atom from its ground state to its excited state of 20.61 eV. The excited He atom collides with a Ne atom in its ground state and excites this atom to the state at 20.66 eV. Lasing action takes place for electron transitions from E3* to E2 in the Ne atoms.

From the data in the figure, show that the wavelength of the red He-Ne laser light is approximately 633 nm.